
Contact Research Organization (CRO)
PT. BMTI also provides comprehensive Contract Research Organization (CRO) services as a strategic partner for the pharmaceutical industry, biotechnology, academia, and research institutions. We support product development processes from basic research to preclinical testing with a standardized, efficient, and reliable scientific approach.
The CRO services we offer include:
Design and Implementation of In Vivo Studies

Pharmacology, efficacy, toxicology, safety, and other supporting studies.
Safety & Toxicology Testing

Including acute, sub-acute, sub-chronic, and chronic toxicity tests, irritation and sensitization studies, as well as evaluation of adverse effects on target organs.
Pharmacology & Efficacy Testing
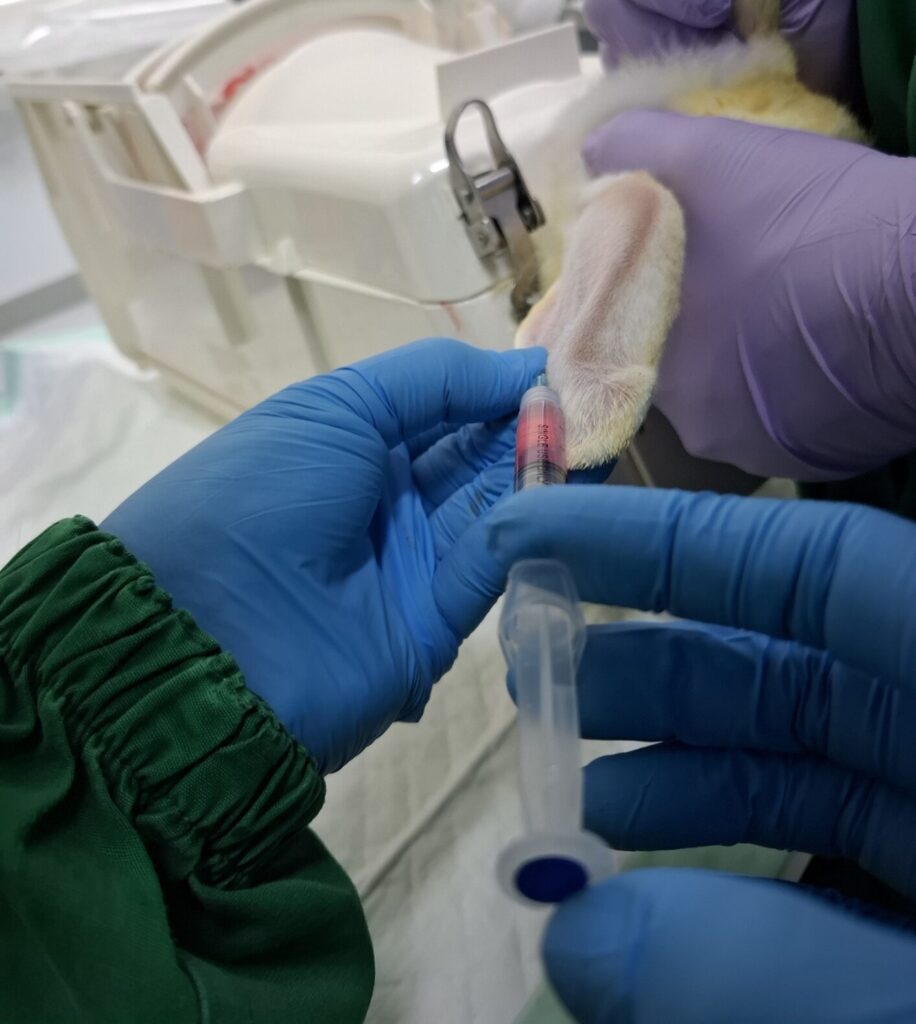
To assess product efficacy in specific disease models, demonstrate biological/therapeutic benefits, and compare with control treatments.
Pharmacokinetic and Distribution Testing
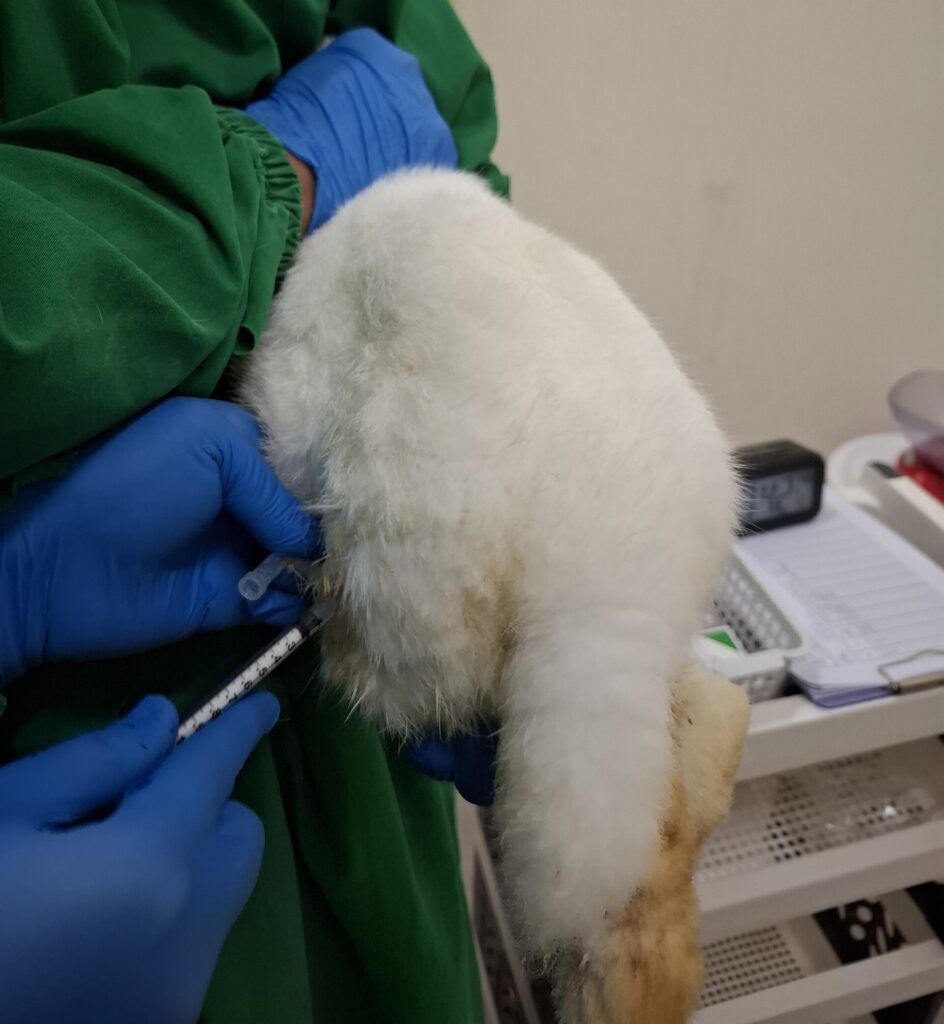
Covers ADME (Absorption, Distribution, Metabolism, Excretion) to understand the behavior of substances within the body.
Immunology & Vaccine Testing
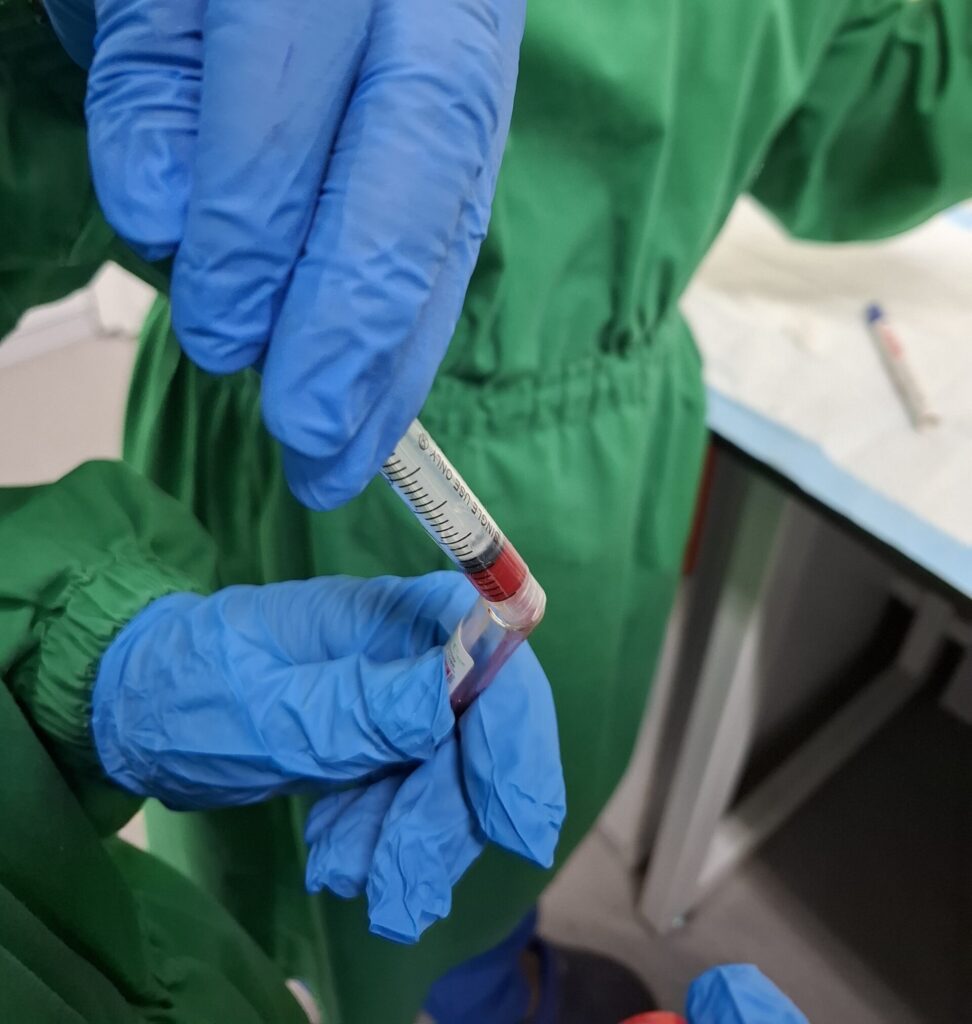
Includes immunogenicity testing, antibody responses, vaccine protection, infectious disease models, and evaluation of immunological safety.
All research is conducted by an experienced team using facilities that support modern laboratory standards, strict SOP implementation, and attention to animal welfare, biosecurity, quality assurance, and scientific integrity. With a professional, measured, and flexible approach, we are ready to be your trusted research partner in generating data that is valid, accurate, and scientifically accountable.
TIM CRO BMTI



Discover more about our Contract Research Organization (CRO) services.
